SPB8 rabbit pAb - 100 μL
The superfamily of high molecular weight serine proteinase inhibitors (serpins) regulate a diverse set of intracellular and extracellular processes such as complement activation, fibrinolysis, coagulation, cellular differentiation, tumor suppression, apoptosis, and cell migration. Serpins are characterized by well-conserved a tertiary structure that consists of 3 beta sheets and 8 or 9 alpha helices (Huber and Carrell, 1989 [PubMed 2690952]). A critical portion of the molecule, the reactive center loop connects beta sheets A and C. Protease inhibitor-8 (PI8; SERPINB8) is a member of the ov-serpin subfamily, which, relative to the archetypal serpin PI1 (MIM 107400), is characterized by a high degree of homology to chicken ovalbumin, lack of N- and C-terminal extensions, absence of a signal peptide, and a serine rather than an asparagine residue at the penultimate position (summary by Bartuski
Distributed by Gentaur
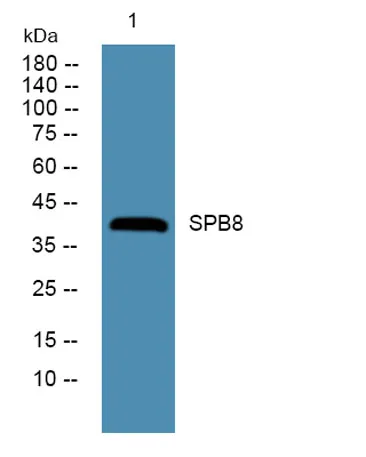
View the Technical Datasheet of the product below

View the Technical Datasheet of the product below